Chronic Hepatitis B (CHB)
Hepatitis B is a viral infection in the liver that can cause both acute and chronic liver disease.1 CHB is diagnosed when HBV infection persists for >6 months.2
Functional Cure is a Treatment Goal Beyond HBV Viral Suppression
CHB is an important health issue in the United States. More than 1 million Americans are living with CHB with majority being foreign-born individuals.3,4,5
HBV is most commonly transmitted from mother to child during pregnancy or birth, while transmission later in life is primarily through exposure to blood infected with HBV.1
NAs (nucleos(t)ide analogs) and Peg-IFN are first-line therapies for CHB in the latest AASLD guideline.6 Current therapies are limited in their ability to achieve functional cure.7
Functional cure is a sustained, undetectable serum HBsAg and HBV DNA with or without seroconversion to anti-HBs after a finite course of therapy.6,8

Unmet Needs in Chronic Hepatitis B
Disease overview
The persistence of chronic HBV infection results from HBV replication and production of viral proteins (such as HBsAg), which in turn promote immune evasion.9
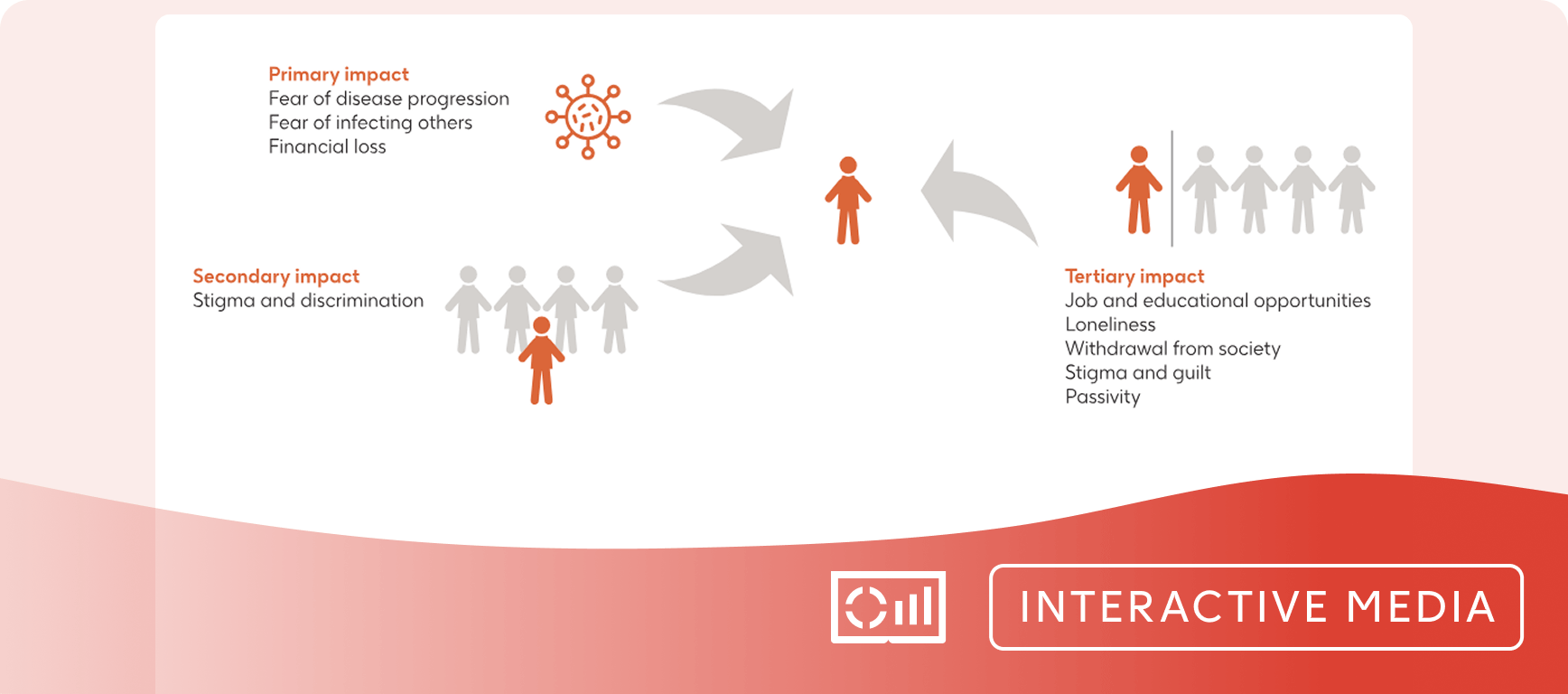
- Patients with CHB are at increased risk of HCC, cirrhosis and death.
- Patients often face stigma/discrimination and may suffer from daily impacts on their well-being and quality of life.10-12
Learn more
Functional Cure
Functional cure is associated with greater reduction in the risk of HCC than HBV DNA suppression alone.13
Barriers to achieving functional cure14,15-17:
- High viral (HBV DNA) and antigen (HBsAg) burden
- Impaired host innate and adaptive immune responses against HBV
- cccDNA and integrated HBV DNA within hepatocytes acting as reservoirs for viral replication and antigen production
Learn more
Quantitative Hepatitis B Surface Antigen Testing
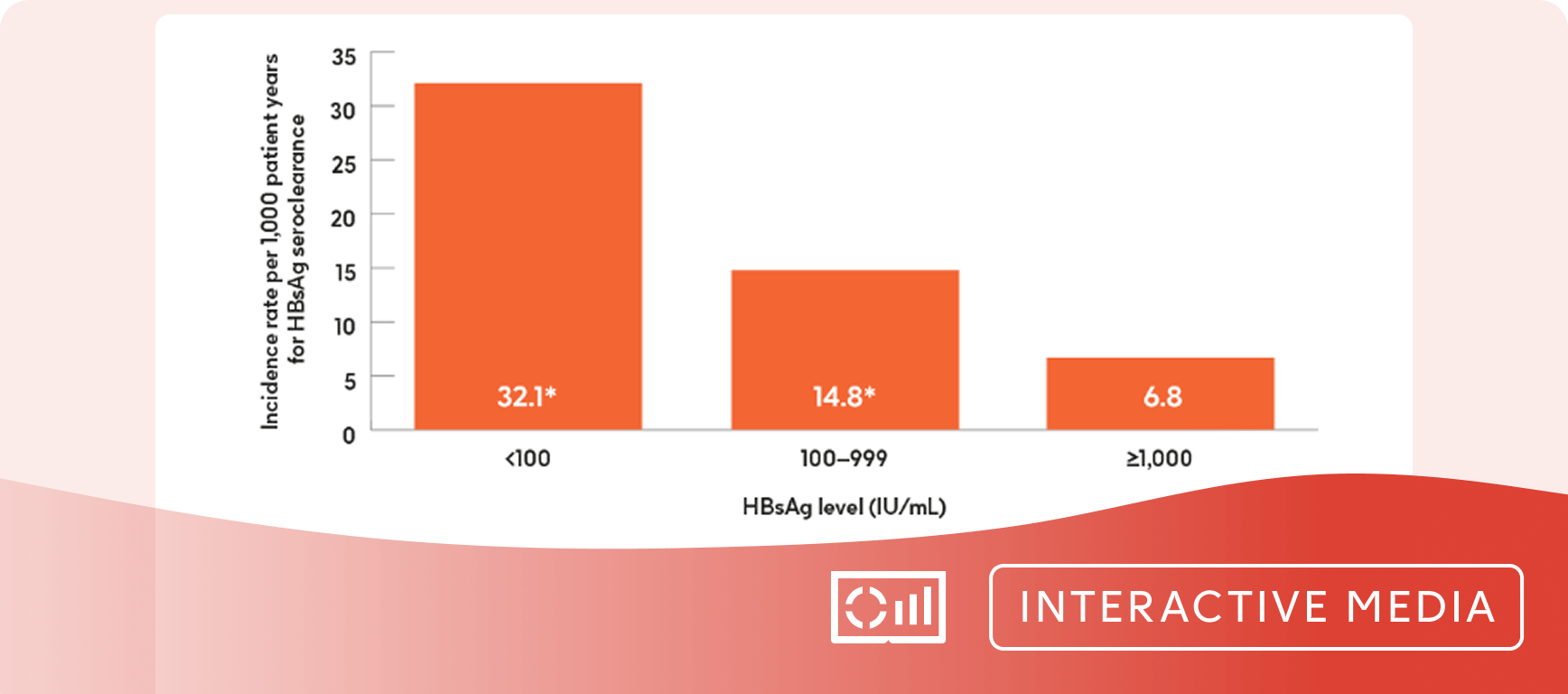
qHBsAg can be used to monitor HBV natural history, aid prognosis prediction, and evaluate/predict treatment response.18-20
- HBsAg loss is associated with improvements in HCC risk, may remove stigma improving patient quality of life, and would consist of a finite therapy duration.13,10,21
Learn more
Learn More About Chronic Hepatitis B
Disease overview
Did you know? Infection in infancy and childhood is associated with greater likelihood of persistence than infection in adulthood due to the immaturity of the immune system.22,23
Functional Cure
Did you know? There is currently no cure for CHB.24
While we can achieve undetectable serum HBV DNA and normal ALT levels with current therapies, the hepatitis B surface antigen remains detectable for most patients: HBsAg loss ≤1% for NAs and 2%–8% for PEG-IFNα after 48 weeks of treatment.11,19
Quantitative Hepatitis B Surface Antigen Testing
Did you know? Assay used to quantitatively measure HBsAg in serum is standardized (IU/mL) and reproducible.25
Laboratories that perform quantitative HBsAg testing include Quest Diagnostics, Labcorp, and Mayo Clinic Laboratories.25
References
- WHO. Hepatitis B. Accessed February 11, 2025. https://www.who.int/news-room/fact-sheets/detail/hepatitis-b
- Hepatitis B Foundation. Acute vs. chronic hepatitis B. Accessed February 11, 2025. https://www.hepb.org/what-is-hepatitis-b/what-is-hepb/acute-vs-chronic
- Wong RJ et al. Hepatology. 2021;74(2):607–626.
- Lim JK et al. Am J Gastroenterol. 2020;115(9):1429–1438.
- The Polaris Observatory Collaborators. Lancet Gastroenterol Hepatol. 2023;8(10):879–907.
- Fanning G.C., et al. Nat Rev Drug Discov 2019;18(11):827-844.
- Li T.Y., et al. World J Gastroenterol 2019;25(27):3527-3537.
- Yip T.C., et al. J Hepatol 2019;70(3):361-370.
- Wong GLH, et al. J Hepatol. 2022;76(6):1249–1262.
- Terrault N.A., et al. Hepatology 2018;67(4):1560-1599.
- European Association for the Study of the Liver. J Hepatol. 2017;67:370–398.
- Ning Q et al. J Viral Hepat. 2019;26(10):1146–1155.
- Chen Y, Tian Z. Front Immunol. 2019;10:2048.
- Dandri M, Petersen J. Infect Drug Resist. 2020;13:3873–3886.
- Wong GL-H, Chan HL-Y. Clin Liver Dis. 2013;2:8–10.
- Wooddell CI, et al. Sci Transl Med. 2017;9:eaan0241.
- Karra VK et al. J Clin Exp Hepatol. 2016;6:209–215.
- Toy M et al. Liver Int. 2022;42(1):16–25.
- Slaets L et al. GastroHep. 2020;2(3):106–116.
- Tsai K.N., et al. Trends Microbiol 2018;26(1):33-42.
- Zhao K., et al. Innovation (Camb) 2020;1(2):100034.
- Testoni B, et al. Semin Liver Dis. 2017;37(3):231–242.
- Lim SG, et al. Aliment Pharmacol Ther. 2021;53:172–182.
- Choi H.S.J., et al. Hepatol Commun 2022;6(5):935-949.
- Martinot-Peignoux M, et al. Liver Int. 2014;34 Suppl 1:97–107.